10
2021.11
疾病的預言:miRNA與帕金森氏症
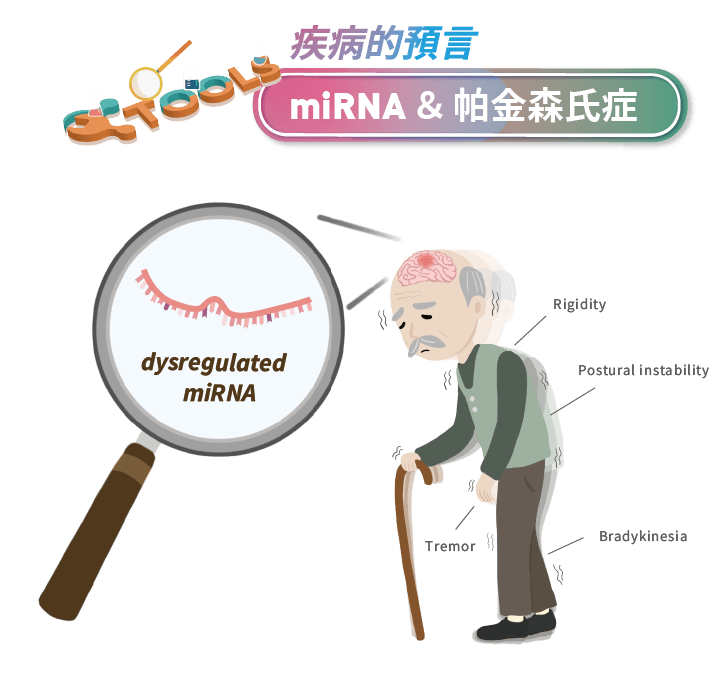
60歲以上族群,估計有1%的人罹患帕金森氏症(Parkinson’s Disease)。帕金森病患會出現運動功能障礙等臨床症狀:靜止性震顫、運動遲緩、僵硬和姿勢不穩。這些病癥源自於黑質(SNc)中多巴胺能(Dopaminergic)神經元的逐漸喪失,導致多巴胺神經遞質信號相對減少;此外,研究發現帕金森氏症患者的神經元中具有大量由α-synuclein蛋白聚集所形成的Lewy體,被認為可能會損害神經元細胞之間傳遞的囊泡運輸或啟動神經炎症等病理機制(Goh et al., 2019)。
臨床上診斷帕金森氏症仍然依賴臨床表徵作為評估的標準,導致診斷的準確性與效率不足。到目前為止,還沒有確切的生物標誌物可以預測或診斷帕金森氏症的病理過程,或監測病患對治療方法的反應。而在過去的十年中,利用RNA測序、晶片和microRNA qPCR分析等技術的研究顯示:miRNAs的表達分布與帕金森氏症有關,且不僅在腦組織中發生改變,在外週體液如腦脊液、血液、甚至唾液和尿液中也有改變。而外週體液中的miRNAs容易通過非侵入式、微侵入式的方式取得並加以分析,探討外週體液中miRNAs表達變化與帕金森氏症之病理進程的關係,對開發帕金森氏症的診斷分子相當具有潛力 (Roser et al., 2019)。

血液及其衍生物是最常被研究的週邊體液。利用PCR陣列分析全血中的miRNA含量,發現了一組差異表達的miRNA,顯著區分帕金森患者和對照的miR-1-3p、miR-22-5p和miR-29a-3p,以及可區分Levodopa/Carbidopa治療組和未治療組的miR-16-2-3p、miR-26a-2-3p和miR30a-5p(Margis et al., 2011)。另在帕金森患者的血清中發現miR-137-3p和miR-124-3p受到顯著地調節(Li et al., 2017)。Ravanidit et al.等報導血清中的miRNAs在特發性和遺傳性帕金森患者之間是不同的,但在不同遺傳形式的帕金森氏症是非常相似的。 在GBA和SNCA突變的帕金森患者中,miR-132-3p (P = 0.083)與hsa-miR-433-3p顯著地上升 , miR-128-3p (P = 0.01)、miR-136-3p、miR-154-5p、miR-323a-3p、(P = 0.055)、miR-382-5p、miR-409-3p、miR-410-3p和miR-485-5p則顯著下降。這表明,這兩種突變都會聚在相同的miRNA上,驅動帕金森氏症的病理過程。

以19例帕金森患者和13例對照組進行微陣列分析,發現18個miRNA在帕金森氏症中發生改變,包括在血清/血漿中發現數個受調控的miRNA,miR-335、miR-374a。miR-199a-3p, miR-199b-3p, miR-126, miR-151-3p, miR-151-5p, miR-29b, miR-147, miR-28-5p, miR-30b, miR-374b, miR-19b, miR-30c, miR-29c, miR-301a, miR-26(Martins et al. , 2011). 後續已RT-qPCR分析顯示,帕金森患者的PBMCs中miR-103a-3p、miR-30b-5p和miR-29a-3p的表達增加(Serafin et al., 2015)

Burgos et al. reporting that the miRNA signature in CSF seems to be slightly more stable (Burgos et al., 2014). The level of miR-132-5p in CSF was observed decreased in PD (Gillardon et al., 2008).
Burgos et al.報導CSF中的miRNA特徵似乎稍微穩定(Burgos et al.,2014)。但另有研究指出,在帕金森氏症中觀察到CSF中miR-132-5p的含量下降(Gillardonet al., 2008)。而針對44名帕金森患者和42名對照組進行定量RT-PCR分析,發現帕金森患者的CSF中miR-200a-3p、miR-542-3p和miR-144-5p是上升,而結果更顯示這些miRNA與疾病階段有高度的相關性(Mo et al., 2016)。另從CSF中分離的外泌體 (exosome),其中miRNAs也顯示與疾病狀態的相關表達。調查這類樣本的研究顯示,miR-1、miR-19b-3p在帕金森氏症中的表達下降,而miR-153、miR-409-3p、miR-10a-5p和let-7g-3p被發現上升。

唾液中miRNA的發現是繼研究帕金森病患的唾液中血氧酶-1(HO1)過度表達的研究之後進行的。由Hyman Schipper領導的團隊先前發現帕金森患者的唾液中,其HO1蛋白明顯升高,因此他們進一步調查了HO1途徑的下游miRNA。他們收集了83名帕金森患者和77名沒有神經系統疾病的對照者的唾液,而後以RT-qPCR分析這些樣本中的miR-153和miR-223的含量,發現帕金森患者的唾液中這兩種miRNA的含量低於對照組(Cressatti et al., 2019年)。
目前,許多研究已經發現周邊miRNAs可做為帕金森氏症和其他相關綜合病症的潛在生物標的物。然而,在不同研究結果之間的重複度很低,且miRNA在病理發展階段的變化還需要更多探討與實際驗證。此外,也需要快速、可靠、易操作和低成本的miRNA檢測方法,才能推動miRNA的研究,盡快找出適合臨床應用的診斷性標的物。

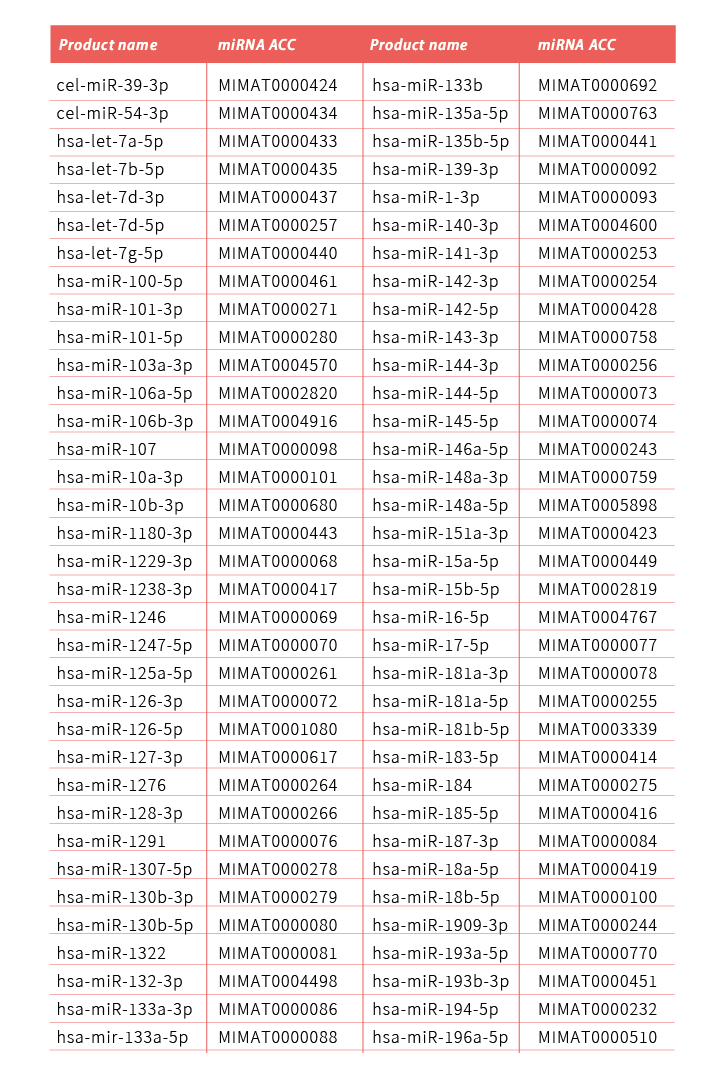
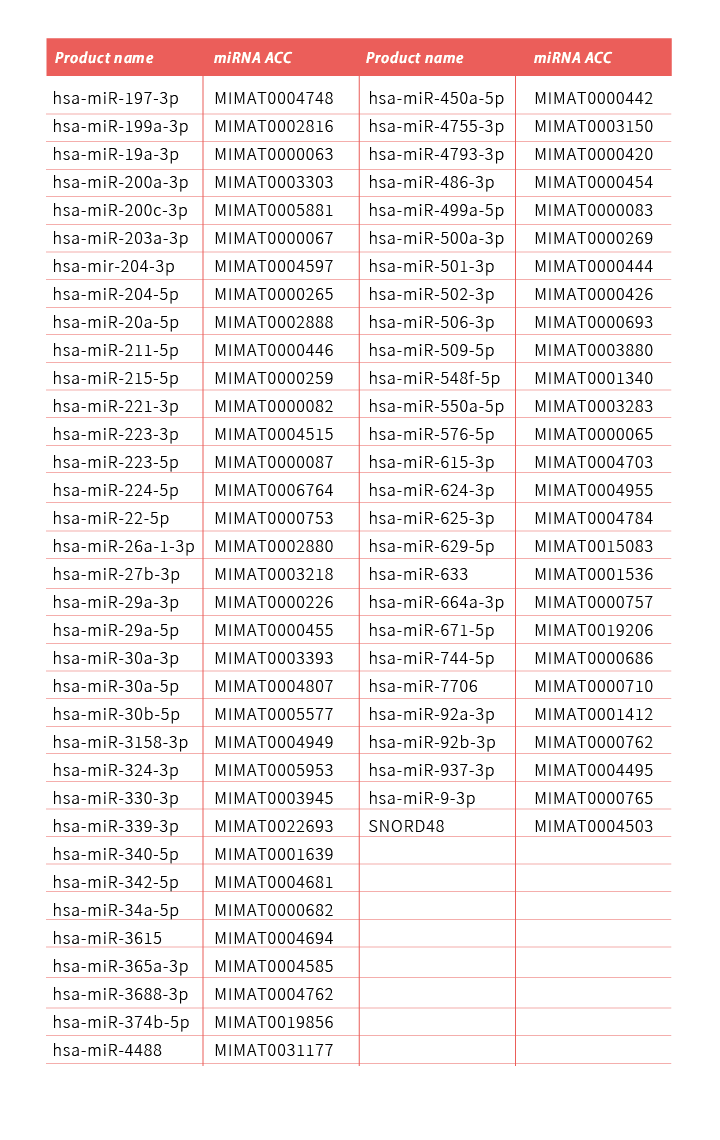
TOOLS推出了全方位的miRNA分析系列產品,包括TOOLS miRNA RT kit (TTH-mi250),TOOLS miRNA RT-qPCR primer/probe set,以及TOOLS Easy 2X probe qPCR mix (TTC-QE13),僅需簡單的2步RT-qPCR實驗流程,即可快速、敏感和特異地分析目標microRNA(miRNA)的表達。其中TOOLS miRNA RT-qPCR引物/探針組都是針對單一miRNA預先設計,專一性高,經過嚴格驗證和品質控制,使用方便簡單,目前已多達200多個miRNA可供選擇,亦涵蓋帕金森氏症研究中報導過的miRNA。TOOLS miRNA RT-qPCR引物/探針組亦可定制設計和生產。

Reference
1.Burgos K, Malenica I, Metpally R, Courtright A, Rakela B, Beach T, Shill H, Adler C, Sabbagh M, Villa S, Tembe W, Craig D, Van Keuren-Jensen K. Profiles of extracellular miRNA in cerebrospinal fluid and serum from patients with Alzheimer's and Parkinson's diseases correlate with disease status and features of pathology. PLoS One. 2014 May 5;9(5):e94839. doi: 10.1371/journal.pone.0094839.
2.Cressatti M, Juwara L, Galindez JM, Velly AM, Nkurunziza ES, Marier S, Canie O, Gornistky M, Schipper HM. Salivary microR-153 and microR-223 Levels as Potential Diagnostic Biomarkers of Idiopathic Parkinson's Disease. Mov Disord. 2020 Mar;35(3):468-477. doi: 10.1002/mds.27935.
3.Gillardon F, Mack M, Rist W, Schnack C, Lenter M, Hildebrandt T, Hengerer B. MicroRNA and proteome expression profiling in early-symptomatic α-synuclein(A30P)-transgenic mice. Proteomics Clin Appl. 2008 May;2(5):697-705. doi: 10.1002/prca.200780025.
4.Goh SY, Chao YX, Dheen ST, Tan EK, Tay SS. Role of MicroRNAs in Parkinson's Disease. Int J Mol Sci. 2019 Nov 12;20(22):5649. doi: 10.3390/ijms20225649.
5.Li N, Pan X, Zhang J, Ma A, Yang S, Ma J. Plasma levels of miR-137 and miR-124 are associated with Parkinson’s disease but not with Parkinson’s disease with depression. Neurol. Sci. 2017, 38, 761–767. doi: 10.1007/s10072-017-2841-9
6.Margis R and Rieder CRM. Identification of blood microRNAs associated to Parkinsonós disease. J. Biotechnol. 2011, 152, 96–101. doi: 10.1016/j.jbiotec.2011.01.023
7.Martins, M., Rosa, A., Guedes, L. C., Fonseca, B. V., Gotovac, K., Violante, S. Convergence of miRNA expression profiling, a-synuclein interacton and GWAS in Parkinson’s disease. PLoS One 2011, 6:e25443. doi: 10.1371/journal. pone.0025443
8.Mo M, Xiao Y, Huang S, Cen L, Chen X, Zhang L, Luo Q, Li S, Yang X, Lin X, Xu P. MicroRNA expressing profiles in A53T mutant alpha-synuclein transgenic mice and Parkinsonian. Oncotarget. 2017 Jan 3;8(1):15-28. doi: 10.18632/oncotarget.13905.
9.Ravanidis S, Bougea A, Papagiannakis N, Maniati M, Koros C, Simitsi AM, Bozi M, Pachi I, Stamelou M, Paraskevas GP, Kapaki E, Moraitou M, Michelakakis H, Stefanis L, Doxakis E. Circulating Brain-enriched MicroRNAs for detection and discrimination of idiopathic and genetic Parkinson's disease. Mov Disord. 2020 Mar;35(3):457-467. doi: 10.1002/mds.27928.
10.Roser AE, Caldi Gomes L, Schünemann J, Maass F, Lingor P. Circulating miRNAs as Diagnostic Biomarkers for Parkinson's Disease. Front Neurosci. 2018 Sep 5;12:625. doi: 10.3389/fnins.2018.00625.
11.Serafin A, Foco L, Zanigni S, Blankenburg H, Picard A, Zanon A, Giannini G, Pichler I, Facheris MF, Cortelli P, Pramstaller PP, Hicks AA, Domingues FS, Schwienbacher C. Overexpression of blood microRNAs 103a, 30b, and 29a in L-dopa-treated patients with PD. Neurology. 2015 Feb 17;84(7):645-53. doi: 10.1212/WNL.0000000000001258.
2.Cressatti M, Juwara L, Galindez JM, Velly AM, Nkurunziza ES, Marier S, Canie O, Gornistky M, Schipper HM. Salivary microR-153 and microR-223 Levels as Potential Diagnostic Biomarkers of Idiopathic Parkinson's Disease. Mov Disord. 2020 Mar;35(3):468-477. doi: 10.1002/mds.27935.
3.Gillardon F, Mack M, Rist W, Schnack C, Lenter M, Hildebrandt T, Hengerer B. MicroRNA and proteome expression profiling in early-symptomatic α-synuclein(A30P)-transgenic mice. Proteomics Clin Appl. 2008 May;2(5):697-705. doi: 10.1002/prca.200780025.
4.Goh SY, Chao YX, Dheen ST, Tan EK, Tay SS. Role of MicroRNAs in Parkinson's Disease. Int J Mol Sci. 2019 Nov 12;20(22):5649. doi: 10.3390/ijms20225649.
5.Li N, Pan X, Zhang J, Ma A, Yang S, Ma J. Plasma levels of miR-137 and miR-124 are associated with Parkinson’s disease but not with Parkinson’s disease with depression. Neurol. Sci. 2017, 38, 761–767. doi: 10.1007/s10072-017-2841-9
6.Margis R and Rieder CRM. Identification of blood microRNAs associated to Parkinsonós disease. J. Biotechnol. 2011, 152, 96–101. doi: 10.1016/j.jbiotec.2011.01.023
7.Martins, M., Rosa, A., Guedes, L. C., Fonseca, B. V., Gotovac, K., Violante, S. Convergence of miRNA expression profiling, a-synuclein interacton and GWAS in Parkinson’s disease. PLoS One 2011, 6:e25443. doi: 10.1371/journal. pone.0025443
8.Mo M, Xiao Y, Huang S, Cen L, Chen X, Zhang L, Luo Q, Li S, Yang X, Lin X, Xu P. MicroRNA expressing profiles in A53T mutant alpha-synuclein transgenic mice and Parkinsonian. Oncotarget. 2017 Jan 3;8(1):15-28. doi: 10.18632/oncotarget.13905.
9.Ravanidis S, Bougea A, Papagiannakis N, Maniati M, Koros C, Simitsi AM, Bozi M, Pachi I, Stamelou M, Paraskevas GP, Kapaki E, Moraitou M, Michelakakis H, Stefanis L, Doxakis E. Circulating Brain-enriched MicroRNAs for detection and discrimination of idiopathic and genetic Parkinson's disease. Mov Disord. 2020 Mar;35(3):457-467. doi: 10.1002/mds.27928.
10.Roser AE, Caldi Gomes L, Schünemann J, Maass F, Lingor P. Circulating miRNAs as Diagnostic Biomarkers for Parkinson's Disease. Front Neurosci. 2018 Sep 5;12:625. doi: 10.3389/fnins.2018.00625.
11.Serafin A, Foco L, Zanigni S, Blankenburg H, Picard A, Zanon A, Giannini G, Pichler I, Facheris MF, Cortelli P, Pramstaller PP, Hicks AA, Domingues FS, Schwienbacher C. Overexpression of blood microRNAs 103a, 30b, and 29a in L-dopa-treated patients with PD. Neurology. 2015 Feb 17;84(7):645-53. doi: 10.1212/WNL.0000000000001258.





