30
2022.05
快速上手!最簡單的DNA萃取方法
提取基因組DNA (genomic DNA, gDNA)是一種常規的分子生物學實驗室技術。gDNA包含生物體的所有遺傳、染色體物質,它編碼生命所必需的所有成分,研究單個基因或對整個基因組進行測序等等實驗[1],是大規模流行病學研究中至關重要的第一步[2, 3]。
隨著這個流感、病毒快速來襲的時代,各種疾病研究中,對於gDNA分析的需求不斷增加,增加了對大量gDNA提取的需求,如何快速有效的萃取高質量的gDNA並且進行下游的分析至關重要。提取gDNA的樣本形式與應用非常多元,如:收集口腔粘膜刮樣,用於檢測口腔生菌數與口腔癌誘發因子;進行深喉痰液與鼻腔採樣,用於檢測呼吸道病毒;從血液樣本中提取gDNA來檢測和監測癌症患者的疾病等[4-6]。
然而傳統的提取gDNA的各種方法一直存在著許多缺點,包括:程序繁雜、時間長、產量低、成本高、品質受損、使用有毒有機溶劑等,並且僅適用於某些類型的組織[3, 7-11]。
圖爾思為快速提取DNA提供了簡單、省時和經濟的方法:EX-TOOL Quick DNA Extraction Solution (TTJ-QS100),此產品簡化許多操作步驟,比傳統的提取方法減少更多步驟和時間,不需使用任何有毒化學品或任何額外的材料,只需要簡單的混和與加熱處理,且只需要3 - 6分鐘即可有效萃取gDNA;實驗證明其所製備的樣本適用於PCR或是qPCR等分子檢測方法學,每批驗證品質穩定,適合分子檢測開發與高通量使用。EX-TOOL Quick DNA Extraction Solution適用於各種樣本,包含各種動物組織、細胞和全血。
隨著這個流感、病毒快速來襲的時代,各種疾病研究中,對於gDNA分析的需求不斷增加,增加了對大量gDNA提取的需求,如何快速有效的萃取高質量的gDNA並且進行下游的分析至關重要。提取gDNA的樣本形式與應用非常多元,如:收集口腔粘膜刮樣,用於檢測口腔生菌數與口腔癌誘發因子;進行深喉痰液與鼻腔採樣,用於檢測呼吸道病毒;從血液樣本中提取gDNA來檢測和監測癌症患者的疾病等[4-6]。
然而傳統的提取gDNA的各種方法一直存在著許多缺點,包括:程序繁雜、時間長、產量低、成本高、品質受損、使用有毒有機溶劑等,並且僅適用於某些類型的組織[3, 7-11]。
圖爾思為快速提取DNA提供了簡單、省時和經濟的方法:EX-TOOL Quick DNA Extraction Solution (TTJ-QS100),此產品簡化許多操作步驟,比傳統的提取方法減少更多步驟和時間,不需使用任何有毒化學品或任何額外的材料,只需要簡單的混和與加熱處理,且只需要3 - 6分鐘即可有效萃取gDNA;實驗證明其所製備的樣本適用於PCR或是qPCR等分子檢測方法學,每批驗證品質穩定,適合分子檢測開發與高通量使用。EX-TOOL Quick DNA Extraction Solution適用於各種樣本,包含各種動物組織、細胞和全血。
- 實驗流程
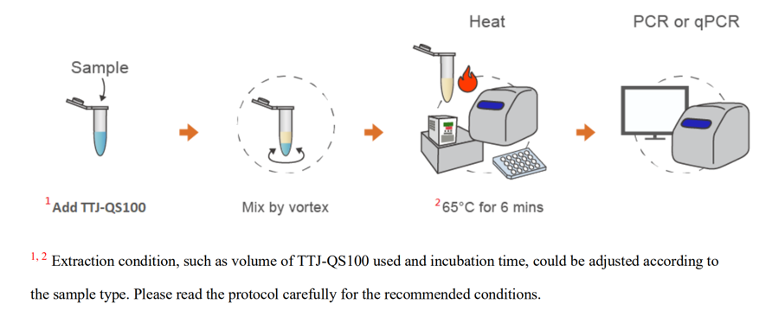
- 產品特色
超簡單:僅需加熱與震盪,即可萃取核酸
省時:實驗證實6分鐘即可有效萃取
應用性廣:實驗證實後端可用於PCR與qPCR,使用方法簡單,極適合高通量樣本處理與分析
- 實驗數據
EX-TOOL Quick DNA Extraction Solution經過驗證,整個實驗只需要3 - 6分鐘即可有效萃取DNA (圖一)。使用EX-TOOL Quick DNA Extraction Solution備置的gDNA樣本相當穩定,儲存穩定性測試,結果顯示含EDTA的人類血液樣本以EX-TOOL Quick DNA Extraction Solution提取,儲存14天後使用於PCR,其結果依然與未存放之樣本一樣(圖二)。另外,亦可使用於各種動物組織樣本,如:常見的模式動物,小鼠的脾臟、心臟和血液(圖三),以及特殊的鸚鵡的血液和羽毛 (羽根),從中提取發生於鸚鵡常見的病毒感染 (圖四、五)。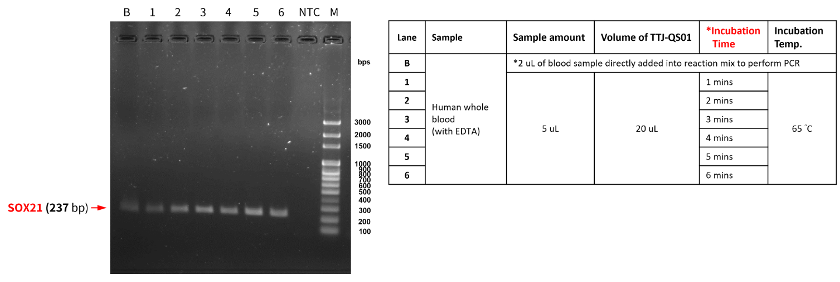
- Figure 1:Genomic DNA of human blood were prepared with EDTA by EX-TOOL Quick DNA Extraction Solution (TTJ-QS01). The samples were extracted with variety of incubation time and then used for PCR amplification using TOOLS 2X SuperGreen PCR Master Mix (TTC-PA31-10) to examine the extraction efficiency by electrophoresis (100V, 1% TAE agarose gel prepared with TOOLS DNA View (TT-DNA01, BIOTOOLS) in 1X TAE buffer (TT-50TA, TOOLS) for 30 minutes. NTC: no template control. M: TOOLS 1kb Ladder (TMG1kb, BIOTOOLS).Figure 1:Genomic DNA of human blood were prepared with EDTA by EX-TOOL Quick DNA Extraction Solution (TTJ-QS01). The samples were extracted with variety of incubation time and then used for PCR amplification using TOOLS 2X SuperGreen PCR Master Mix (TTC-PA31-10) to examine the extraction efficiency by electrophoresis (100V, 1% TAE agarose gel prepared with TOOLS DNA View (TT-DNA01, BIOTOOLS) in 1X TAE buffer (TT-50TA, TOOLS) for 30 minutes. NTC: no template control. M: TOOLS 1kb Ladder (TMG1kb, BIOTOOLS).
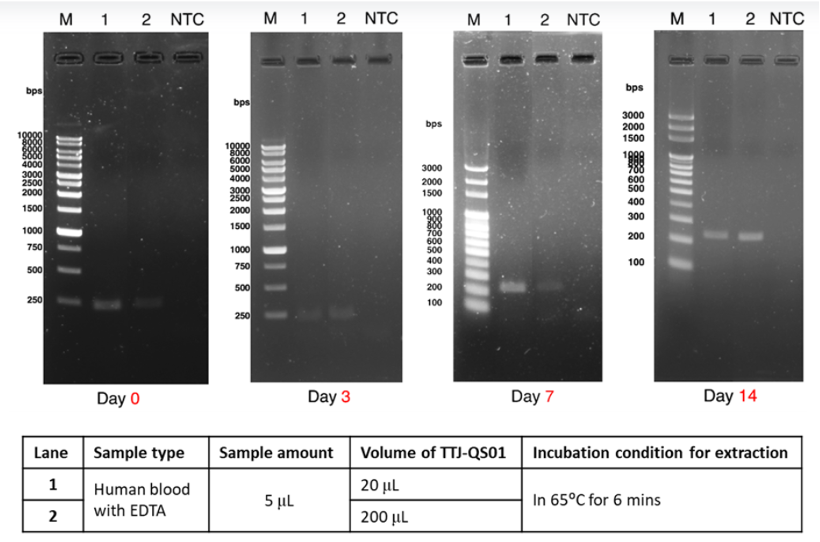
- Figure 2:Genomic DNA from human blood with EDTA were prepared with EX-TOOL Quick DNA Extraction Solution (TTJ-QS01). The extracted samples further were taken to perform PCR amplification using TOOLS 2X SuperGreen PCR Master Mix (TTC-PA31-10) immediately or after stored for 3, 7, 14 days, The PCR products of SOX21, 237 bp, in every sample were then examined with electrophoresis (100V, 1% TAE agarose gel prepared with TOOLS DNA View (TT-DNA01, BIOTOOLS) in 1X TAE buffer (TT-50TA, TOOLS) for 30 minutes. DNA size was referenced with DNA Ladder. NTC: no template control.
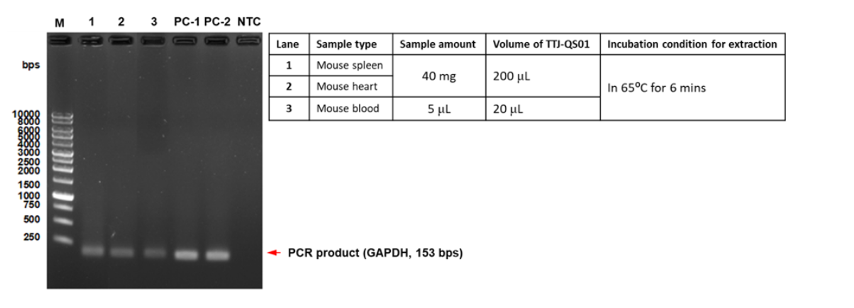
- Figure 3:Genomic DNA from mouse spleen, heart, kidney, blood are prepared with EX-TOOL Quick DNA Extraction Solution (TTJ-QS01). The extracted samples were then used for PCR amplification using TOOLS 2X SuperGreen PCR Master Mix (TTC-PA31-10) examine the extraction efficiency with electrophoresis (100V, 1% TAE agarose gel prepared with TOOLS DNA View (TT-DNA01, BIOTOOLS) in 1X TAE buffer (TT-50TA, TOOLS) for 30 minutes. DNA size was referenced with TOOLS 1kb Ladder (TMG1kb, BIOTOOLS). PC-1: mouse cell cDNA diluted with TTJ-QS01, PC-2: mouse cell cDNA diluted with ddH2O, NTC: no template control.
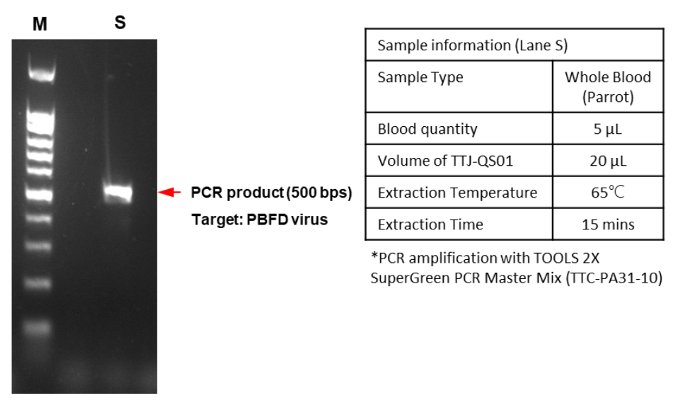
- Figure 4:Genomic DNA from whole blood of parrot was prepared with EX-TOOL Quick DNA Extraction Solution (TTJ-QS01). The extracted sample was then used for PCR amplification and electrophoresis to examine the extraction efficiency. The amplicon size was referenced as 500 bp with DNA ladder (lane M).
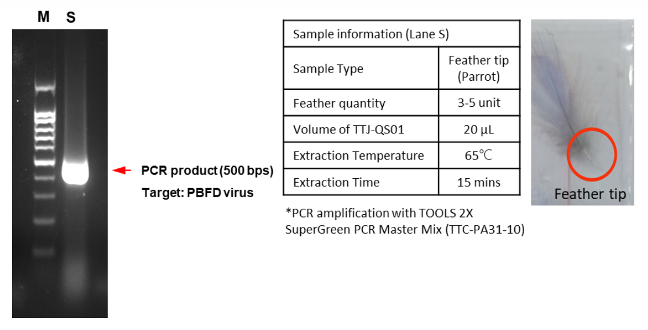
- Figure 5:Genomic DNA from feather tip was prepared with EX-TOOL Quick DNA Extraction Solution (TTJ-QS01). The extracted sample was then used for PCR amplification and electrophoresis to examine the extraction efficiency. The amplicon size was referenced as 500 bp with DNA ladder (lane M).
Reference
1. Greathouse, K.L., R. Sinha, and E. Vogtmann, DNA extraction for human microbiome studies: the issue of standardization. Genome Biol, 2019. 20(1): p. 212.
2. Koshy, L., A.L. Anju, S. Harikrishnan, V.R. Kutty, V.T. Jissa, I. Kurikesu, P. Jayachandran, A. Jayakumaran Nair, A. Gangaprasad, G.M. Nair, and P.R. Sudhakaran, Evaluating genomic DNA extraction methods from human whole blood using endpoint and real-time PCR assays. Mol Biol Rep, 2017. 44(1): p. 97-108.
3. Koh, C.M., Chapter Thirteen - Isolation of Genomic DNA from Mammalian Cells, in Methods in Enzymology, J. Lorsch, Editor. 2013, Academic Press. p. 161-169.
4. Ghatak, S., R.B. Muthukumaran, and S.K. Nachimuthu, A simple method of genomic DNA extraction from human samples for PCR-RFLP analysis. J Biomol Tech, 2013. 24(4): p. 224-31.
5. Leest, P.V., P.A. Boonstra, A.T. Elst, L.C.V. Kempen, M. Tibbesma, J. Koopmans, A. Miedema, M. Tamminga, H.J.M. Groen, A.K.L. Reyners, and E. Schuuring, Comparison of Circulating Cell-Free DNA Extraction Methods for Downstream Analysis in Cancer Patients. Cancers (Basel), 2020. 12(5).
6. Cheng, T.-H., S.-P. Chen, T.-C. Lu, W.-C. Chen, J.-S. Sher, and Y.-S.J.J.o.M.S. Shieh, Optimal DNA extraction from buccal swab samples. 2010. 30(4): p. 149-154.
7. Angelini, A., C. Di Febbo, A. Rullo, C. Di Ilio, F. Cuccurullo, and E. Porreca, New method for the extraction of DNA from white blood cells for the detection of common genetic variants associated with thrombophilia. Pathophysiol Haemost Thromb, 2002. 32(4): p. 180-3.
8. Elgort, M.G., M.G. Herrmann, M. Erali, J.D. Durtschi, K.V. Voelkerding, and R.E. Smith, Extraction and amplification of genomic DNA from human blood on nanoporous aluminum oxide membranes. Clin Chem, 2004. 50(10): p. 1817-9.
9. Chen, F., J. Ye, C. Chio, W. Liu, J. Shi, and W. Qin, A simplified quick microbial genomic DNA extraction via freeze-thawing cycles. Mol Biol Rep, 2020. 47(1): p. 703-709.
10. Wang, T.Y., L. Wang, J.H. Zhang, and W.H. Dong, A simplified universal genomic DNA extraction protocol suitable for PCR. Genet Mol Res, 2011. 10(1): p. 519-25.
11. Guha, P., A. Das, S. Dutta, and T.K. Chaudhuri, A rapid and efficient DNA extraction protocol from fresh and frozen human blood samples. J Clin Lab Anal, 2018. 32(1).








